High-Precision Radiocarbon Dating
Accelerator Mass Spectrometry (AMS) is a form of mass spectrometry that accelerates ions to extraordinarily high kinetic energies before analysis. In radiocarbon dating, AMS directly counts individual 14C atoms rather than measuring their radioactive decay, dramatically improving precision and reducing sample size requirements.
Revolutionary Advancement
AMS revolutionized radiocarbon dating by reducing sample size requirements from grams to milligrams, enabling dating of precious artifacts and microscopic samples. It also extended the practical dating range and improved precision through direct atom counting rather than decay counting.
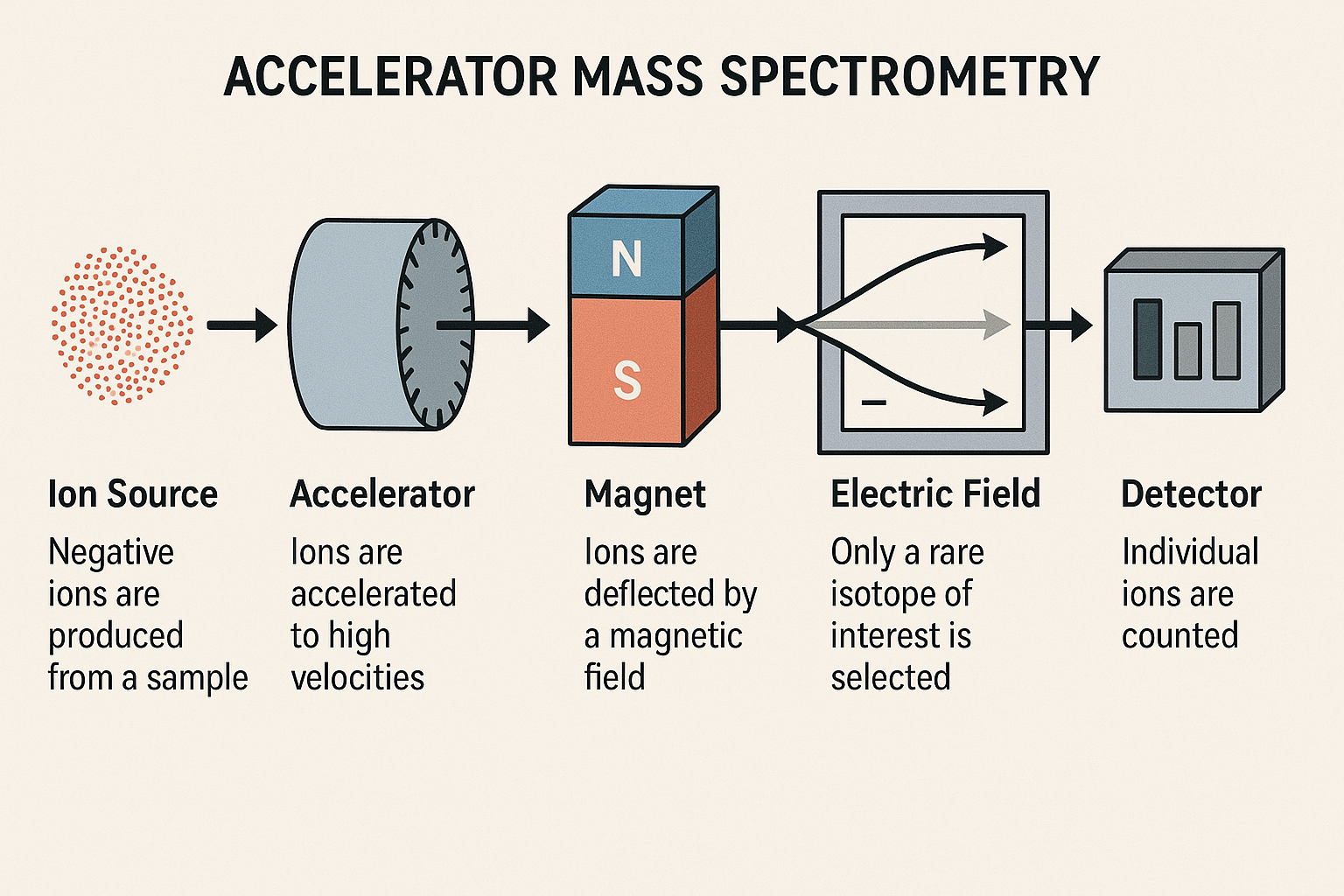
The AMS Process
AMS dating involves sophisticated sample preparation and measurement techniques:
Samples are cleaned and converted to carbon dioxide, then reduced to graphite targets. This process removes contaminants and concentrates carbon for measurement.
Graphite targets are bombarded with cesium ions, creating negative carbon ions (C⁻) that are extracted and accelerated.
Ions are accelerated to millions of electron volts, passed through a gas stripper that removes electrons, creating highly energetic positive ions.
Magnetic and electric fields separate ions by mass and energy. Detectors count individual 14C, 13C, and 12C atoms to determine isotope ratios.
AMS vs. Conventional Radiocarbon Dating
| Aspect | Conventional Dating | AMS Dating |
|---|---|---|
| Sample Size | 1-10 grams | 1-10 milligrams |
| Measurement | Beta decay counting | Direct atom counting |
| Precision | ±50-100 years | ±20-40 years |
| Measurement Time | Days to weeks | Minutes to hours |
| Age Range | ~50,000 years | ~60,000 years |
Applications of AMS Dating
AMS has opened new frontiers in radiocarbon dating:
1. Archaeology
Dating precious artifacts, ancient manuscripts, cave paintings, and human remains with minimal sample destruction. Single seeds, textile fibers, and microscopic organic remains can now be dated.
2. Art Authentication
Verifying the age of paintings, sculptures, and manuscripts by dating organic components like wood, canvas, or parchment with minimal damage to the artwork.
3. Forensic Science
Dating recent organic materials in criminal investigations, including identifying when materials were last alive or when synthetic materials were produced.
4. Climate Research
Dating individual foraminifera, pollen grains, and organic compounds in sediment cores to reconstruct high-resolution climate records.
5. Biomedical Research
Tracing carbon-14 labeled compounds through biological systems and dating biological samples with extreme precision.
Limitations and Considerations
Despite its advantages, AMS dating has limitations:
Cost and Accessibility
AMS facilities are expensive to build and maintain, making measurements more costly than conventional radiocarbon dating. Only specialized laboratories can perform AMS dating.
Contamination Sensitivity
Small sample sizes make AMS measurements extremely sensitive to modern carbon contamination. Rigorous cleaning protocols and background corrections are essential.
Calibration Requirements
Like all radiocarbon dating, AMS ages must be calibrated against tree-ring chronologies to account for atmospheric 14C variations over time.
Reservoir Effects
Marine and freshwater samples may show apparent ages different from atmospheric carbon due to reservoir effects, requiring specific corrections.
Conclusion
Accelerator Mass Spectrometry has transformed radiocarbon dating, enabling precise dating of tiny samples and expanding the range of materials that can be analyzed. Its impact extends across archaeology, art history, climate science, and forensics.
AMS Advantages
- Minimal sample destruction
- Enhanced precision and accuracy
- Rapid measurement times
- Extended dating range
- Reduced contamination risks
- Ability to date previously undatable samples
Future Developments
Ongoing improvements in AMS technology continue to push the boundaries of radiocarbon dating. Advances in sample preparation, accelerator design, and detection systems promise even greater precision and smaller sample requirements in the future.