The Science Behind Radiocarbon Dating
Radiocarbon dating (also known as carbon-14 dating or C-14 dating) is a method for determining the age of organic materials up to approximately 50,000 years old. The technique was developed by Willard Libby and his colleagues at the University of Chicago in 1949, for which Libby was awarded the Nobel Prize in Chemistry in 1960.
How It Works
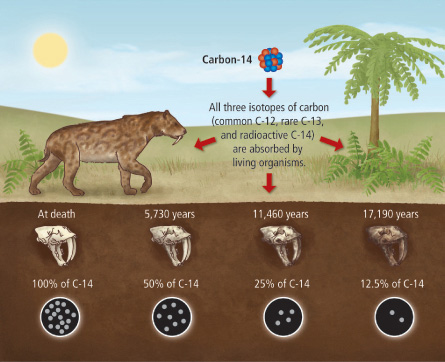
Radiocarbon dating relies on the constant decay of carbon-14, a radioactive isotope of carbon that is continuously produced in Earth's upper atmosphere when cosmic rays interact with nitrogen atoms. Living organisms absorb carbon-14 through the carbon cycle (via photosynthesis for plants and consumption for animals). When an organism dies, it stops exchanging carbon with the environment, and the carbon-14 begins to decay with a half-life of 5,730 ± 40 years.
Modern Measurement Techniques
Since its inception, radiocarbon dating has evolved significantly with advances in technology. Today, there are two primary methods used for measuring carbon-14 content:
AMS directly counts carbon-14 atoms rather than waiting for them to decay. This method requires much smaller samples (0.5-1 mg) compared to conventional methods, making it possible to date tiny samples like seeds, textiles, and paper.
Precision: ±30-50 years for samples less than 10,000 years old.
LSC measures beta particles emitted during radioactive decay. It requires larger samples (several grams) but is still widely used due to its lower cost compared to AMS.
Precision: ±100-200 years for samples less than 10,000 years old.
| Characteristic | AMS | Liquid Scintillation |
|---|---|---|
| Sample size | 0.5-1 mg | 1-5 g |
| Precision | ±30-50 years | ±100-200 years |
| Measurement time | 30-40 minutes | 1-3 days |
| Cost per sample | $400-1000 | $200-400 |
| Practical age limit | ~50,000 years | ~35,000 years |
Calibration and Precision
Raw radiocarbon dates need to be calibrated to account for variations in atmospheric carbon-14 over time. These variations are caused by changes in Earth's magnetic field, solar activity, and more recently, human activities like fossil fuel burning and nuclear testing.
Calibration Curves
Scientists have developed precise calibration curves that convert raw radiocarbon ages into calendar dates. These curves are based on:
- Tree Rings (Dendrochronology): Annual growth rings from long-lived trees like bristlecone pines and European oaks provide a record going back more than 13,900 years.
- Marine Records: Coral records and foraminifera in varved sediments extend the record to approximately 50,000 years.
- Speleothems: Cave formations dated by uranium-thorium methods provide additional calibration points.
- Lake and Marine Sediments: Varves (annual layers in lake sediments) offer additional calibration data.
IntCal20: The Latest Calibration Standard
The most recent internationally agreed calibration curve, IntCal20, was published in 2020. This update extended the calibration curve to 55,000 years BP and significantly improved resolution, particularly in the older ranges. The IntCal20 calibration represents a collaboration of dozens of international laboratories and incorporates over 15,000 measurements from tree rings, lake and marine sediments, cave formations, and corals.
Recent advances in calibration have significantly improved the precision of radiocarbon dating:
| Sample Age | IntCal04 Uncertainty (95% confidence) | IntCal20 Uncertainty (95% confidence) | Improvement |
|---|---|---|---|
| 2,000 BP | ±80 years | ±36 years | 55% reduction |
| 10,000 BP | ±120 years | ±76 years | 37% reduction |
| 30,000 BP | ±450 years | ±215 years | 52% reduction |
| 45,000 BP | ±1500 years | ±650 years | 57% reduction |
Interdisciplinary Reliability
The reliability of radiocarbon dating has been extensively verified through cross-checking with other independent dating methods. This interdisciplinary verification is one of the strongest arguments for its accuracy.
1. Dendrochronology (Tree-Ring Dating)
Tree rings provide an absolute chronology that matches radiocarbon dates with remarkable accuracy. Studies comparing radiocarbon dates with tree-ring sequences show agreement to within ±20 years for samples up to 11,000 years old. The International Tree-Ring Database contains over 4,000 chronologies from 6 continents that have been used to verify radiocarbon dates.
2. Uranium-Thorium Dating
Also known as U-series dating, this method has been used to date cave formations (speleothems) that also contain organic material suitable for radiocarbon dating. A 2018 study in Science examined 23 cave sites across Europe and Asia, finding agreement between uranium-thorium and calibrated radiocarbon dates within ±150 years over a 40,000-year range.
3. Varve Chronology
Annual sediment layers (varves) in lakes provide another independent chronology. The Cariaco Basin off Venezuela contains a 15,000-year varve sequence that correlates with radiocarbon dates to within ±50 years. Similarly, Lake Suigetsu in Japan has a continuous 52,800-year varve record that closely matches calibrated radiocarbon ages.
4. Ice Core Dating
Greenland and Antarctic ice cores contain annual layers that can be counted back tens of thousands of years. Trapped air bubbles in these ice cores contain organic materials that can be radiocarbon dated. The GRIP and GISP2 ice cores from Greenland show agreement with radiocarbon dates to within ±100 years over the past 40,000 years.
5. Archaeological Contexts
Radiocarbon dates from historical contexts with known ages provide strong verification. For example, Egyptian artifacts with known historical dates have been radiocarbon dated, showing agreement within the expected margin of error. The tomb of Tutankhamun (c. 1325 BCE) contained materials that were radiocarbon dated to 1320 ± 40 BCE, confirming the method's accuracy.
Case Studies in Interdisciplinary Verification
Case Study 1: Shroud of Turin
In 1988, three independent laboratories (University of Arizona, Oxford University, and ETH Zurich) performed radiocarbon dating on the Shroud of Turin using AMS. All three labs obtained consistent results placing the cloth's origin between 1260-1390 CE, contrary to claims that it was from the time of Jesus. This date aligned with historical records of the shroud's first appearance in the 1350s and with art historical analyses of the weaving technique.
Case Study 2: Ötzi the Iceman
When the mummified body of a man was discovered in the Alps in 1991, radiocarbon dating placed his death at 5,200 ± 40 years ago. This date has been independently verified through:
- Dendrochronological dating of his wooden axe handle
- Typological analysis of his copper axe and flint tools
- Archaeological context of similar artifacts from the Copper Age
- DNA analysis showing genetic markers consistent with Neolithic European populations
Case Study 3: Chauvet Cave Paintings
The discovery of the Chauvet Cave in France revealed sophisticated cave art initially thought to be around 15,000-17,000 years old based on stylistic analysis. However, radiocarbon dating of charcoal pigments and torch marks yielded dates of 30,000-32,000 years BP, much older than expected. These dates were subsequently confirmed through:
- Uranium-thorium dating of overlying calcite formations
- Thermoluminescence dating of heated cave floor sediments
- Optically stimulated luminescence dating of sediment layers
This case demonstrated radiocarbon dating's ability to challenge existing archaeological assumptions with accurate data.
Statistical Reliability and Quality Assurance
International Intercomparison Studies
Radiocarbon laboratories worldwide participate in regular intercomparison exercises to ensure consistency and accuracy. Recent examples include:
- SIRI (Sixth International Radiocarbon Intercomparison): Completed in 2013, involved 72 laboratories testing identical samples of varying ages. Results showed agreement within statistical margins for over 95% of measurements.
- TIRI/FIRI (Third/Fourth International Radiocarbon Intercomparison): These studies showed that AMS laboratories typically achieve an uncertainty of 2-3‰ (equivalent to ±16-24 years) for modern samples.
| Intercomparison Study | Year | Labs Participating | Agreement Rate |
|---|---|---|---|
| SIRI | 2013 | 72 | 95% |
| VIRI | 2007-2010 | 69 | 93% |
| FIRI | 1999-2001 | 92 | 90% |
| TIRI | 1995 | 74 | 88% |
Laboratory Standards and Procedures
Modern radiocarbon dating employs rigorous quality control measures:
- Regular testing of international standards like Oxalic Acid II (NIST SRM 4990C)
- Analysis of background samples (materials with no measurable C-14)
- Process blanks to detect any contamination during sample preparation
- Analysis of samples of known age to confirm accuracy
- Duplicate measurements to assess precision
These procedures ensure that laboratories maintain a precision of ±20-30 years for samples less than 5,000 years old and ±100-400 years for samples approaching 40,000 years old.
Limitations and Considerations
While radiocarbon dating is a powerful and reliable technique, it does have limitations that scientists carefully account for:
Age Range Limitations
The practical limit for radiocarbon dating is approximately 50,000 years. After about 8-10 half-lives, the amount of remaining carbon-14 becomes too small to measure accurately. For older samples, other radiometric dating methods (such as potassium-argon or uranium-series) must be used.
Sample Contamination
Contamination with modern or ancient carbon can skew results. Modern laboratory protocols include rigorous pretreatment methods to remove contaminants:
- Acid-Base-Acid (ABA): Sequential treatment with acid, base, and acid again to remove carbonates and humic substances
- ABOX-SC: Advanced oxidation pretreatment for samples approaching the radiocarbon limit
- Ultrafiltration: Removal of degraded collagen fragments from bone samples
Reservoir Effects
Marine organisms and those in lakes can incorporate "old carbon" due to the marine reservoir effect, making them appear older than they actually are. Regional reservoir corrections have been developed through research:
- Global marine offset: approximately 400 years
- Local variations (ΔR values) range from -100 to +1500 years depending on ocean circulation
- Freshwater reservoir effects can range from 200-2000 years depending on the water body
Dietary Considerations
The radiocarbon date of human and animal remains can be affected by diet, particularly consumption of marine or freshwater resources. Stable isotope analysis (δ13C and δ15N) is routinely performed alongside radiocarbon dating to assess and correct for dietary effects.
Conclusion: The Reliability of Radiocarbon Dating
Radiocarbon dating stands as one of the most thoroughly tested and verified scientific techniques in modern research. Its reliability is established through:
- Multiple independent verification methods that consistently confirm its accuracy across disciplines
- Rigorous international standardization ensuring consistency between laboratories worldwide
- Continuous improvement in precision through advances in measurement technology and calibration
- Transparent acknowledgment and correction of known limitations and potential sources of error
With proper sample selection, pretreatment, and calibration, radiocarbon dating provides a reliable chronological framework that has revolutionized our understanding of archaeology, paleontology, geology, and climate science. The interdisciplinary consilience of radiocarbon dates with other independent dating methods provides compelling evidence for both the accuracy of the technique and the antiquity of Earth's history.